Protocol Information for Clinicians & Researchers
As our partners in supporting our Remission Biome self-experimenters, we are grateful for your interest in learning more about our protocol to support patients with ME/CFS & Long COVID on their quest for an improved outcome with their symptoms and function. This page will function as a source of updated information and a place to answer frequently asked questions. In addition, we have a downloadable document that we send to all clinicians overseeing patients in our current cohorts. We will be making this available in the near future to clinicians who request this information from us.
Sudden, dramatic remissions from ME/CFS and Long COVID after taking antibiotics have been reported by clinicians, followed by an improved baseline and associated quality of life. The most common antibiotic to cause temporary remissions with subsequent baseline increase is AmoxiClav. RemissionBiome is a research project that has been able to purposely trigger these temporary remissions in ~80% of patients in a small cohort using the 6 phase protocol detailed below.
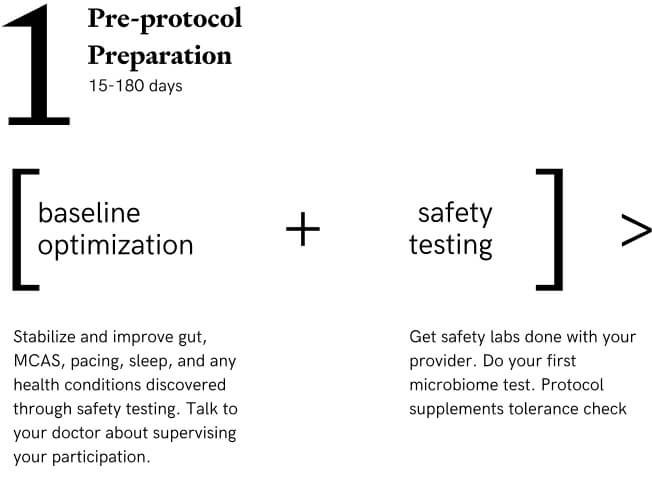
Stage 1: Pre-Protocol Baseline Optimization
Safety Lab Testing (these are standard tests available to doctors to run and most are done with participant’s health care provider), microbiome testing, cognitive test at home, stabilize gut, MCAS, sleep, pacing and optimize individual baseline to ensure best chances for the protocol to work. This stage of the protocol is 100% personalized, allowing us to account for the variation that exists between participants. The timeline for each participant is unpredictable. Participants start symptom tracking. Expected range is 15-180 days.
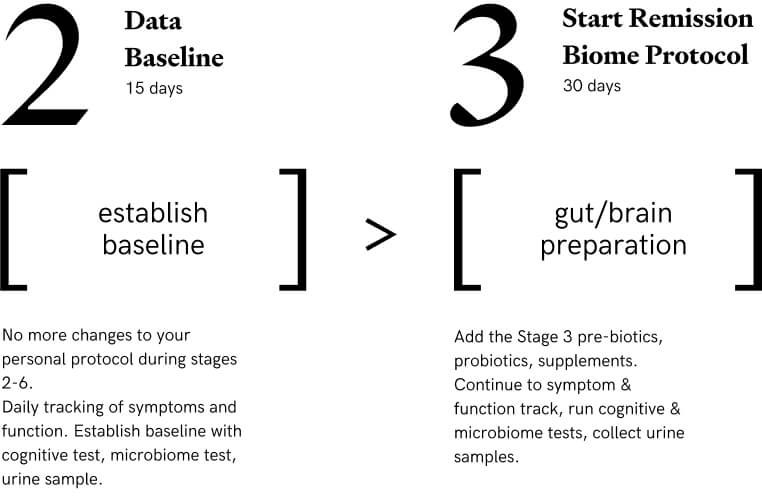
Stage 2: Establish Baseline
Participants commit to making no additional changes to their personal protocols for the remainder of the stages. They track symptoms, test their microbiomes and cognition to establish their baselines. 15 days
Stage 3: Gut/Brain Preparation
Participants start taking protocol supplements, neuro-antiinflammatories, prebiotics, and probiotics on top of their personal protocols. Participants do vagal stimulation and yoga nidra daily to improve their gut brain axis. They also establish their baseline through testing and tracking prior to moving to Stage 4. 30 days
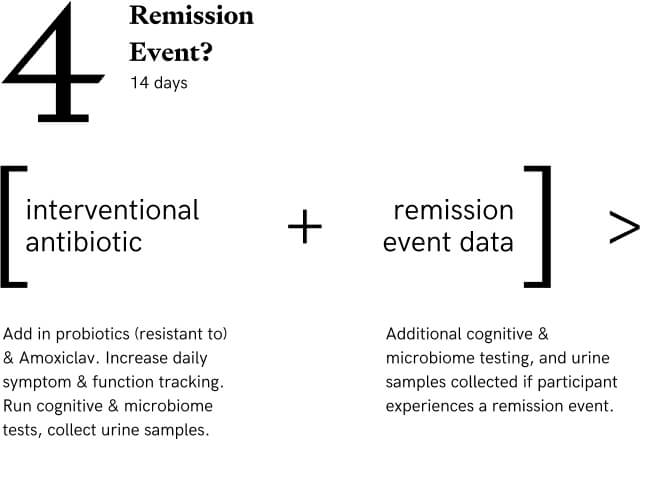
Stage 4: Interventional Antibiotic (Amoxiclav)
Participants add in additional probiotics in addition to the ones in Stage 3 and a 14 day course of Amoxiclav (875 mg Amoxicillin/125 mg Clavulanic Acid) TID. Cycling of symptoms is expected and some will experience remission events (we do not know for sure if participants need to experience a remission event to end up with a higher baseline on the other side - more data will reveal this.) Tracking and testing (urine samples collected, microbiome samples collected, cognitive testing done at home) is collected during this stage. 14 days
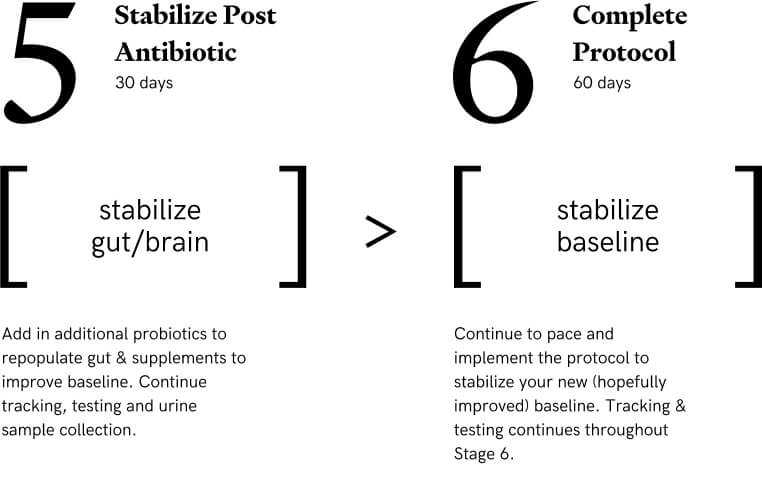
Stage 5: Re-Establish Baseline - Stabilize Gut/Brain
Additional 30 days of an antiinflammatory antibiotic, some changes to the probiotics and a continuation of many of the previous items to repopulate and stabilize the gut and brain. Additional cognitive testing, symptom tracking, microbiome testing will be performed. 30 days
Stage 6: Stabilize Baseline
This stage is to help stabilize an increased baseline. We hypothesize that taking time to continue pacing and continue with some of the prebiotics, probiotics, and supplements even if the participant has achieved an increased baseline is important to stabilize the baseline for the future. At the end of this Stage participants can continue to use some of the supplements and probiotics going forward and can make changes to their own personal protocols at this point. 60 days
We’ve learned a lot working with 50 more participants as part of our first large cohort to continue testing and improving the protocol. Stage 1 requires a lot more time than we expected and is when working directly with you is vital. We’ll be sharing more specific lessons we’ve learned and reasons why we’ve included all of the tests we ask you to run in this stage in the near future.
We currently expect to see improvements in baseline at the end of the protocol for potentially 80% of participants. Thai is based on the small group we ran in 2023 and you learn more here on our Protocol Results page. As results roll in for our current groups, all in different stages of the protocol as we write this, we’ll share more with you. Our focus on this page is to empower you with the data, that can inform your decisions as you help your patients navigate the protocol. Here’s a brief overview of each stage with a bit more information than the graphic above.
We’ll be looking at multiple data points:
Primary data point is the end of Stage 6 which is 134 days after beginning Stage 2 where participants are establishing their personal baseline prior to beginning the protocol where we measure the participants' changes in comparison to their baselines.
Secondary data point is evaluating symptom tracking, urine samples with metabolomics testing, cognitive testing all in comparison to baselines if participants experience remission events so that we can better understand what is happening.
Tertiary data point is the end of the Stage 3 which is the first 30 days of using supplements, prebiotics and probiotics to seed the gut prior to using the Amoxiclav in Stage 4.
Quaternary data point is evaluating the improvements participants are making in Stage 1 as we have seen that many of the participants have experienced such significant improvements during this stage that it may be sufficient for many future participants to just do Stage 1.
Clarifying a few points:
There is a list of probiotics, prebiotics, and supplements for each of the stages 3-6 of the protocol. In addition, the participants’ health care providers prescribe two different antibiotics for stages 4 and 5.
The data we focus on is symptom and function tracking looking for trends and changes, cognitive tests run numerous times, microbiome samples collected at home and evaluated by Biomesight, urine samples collected and frozen so that we can evaluate them in the case of remission events.
Our symptom tracking is extensive and includes daily tracking of all treatments, physical and cognitive symptoms, any viruses, overall wellness scores many times a day and a daily overall cognitive and physical function score. Most participants with health trackers are sharing their daily data as well and we hope to add in standard health trackers in the future.
What makes our project very different from most research currently being done is that we are testing a multi treatment protocol and not just a single intervention. The first stage of the protocol is personalized and prepares participants rather than expecting participants to already meet metrics they may not be able to meet on their own. We are also collecting daily symptom tracking throughout the protocol which is above and beyond what most researchers are able to collect.
We plan to make all of our data and results public so that everyone can utilize what we learn either to use the protocol for themselves or for researchers to follow up on potential leads in their studies. We also look forward to collaborating with researchers so that we can collectively speed up getting effective treatments to patients around the world.
